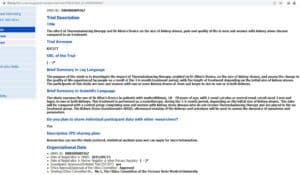
Clinical Trial Registration, No: DRKS00009367
Study title: The effect of Thermobalancing therapy with Dr Allen’s Device on the size of kidney stones, pain and quality of life in men and women with kidney stone disease compared to no treatment
This clinical trial was registered with the DRKS Register in Germany in 2015 with the registration number DRKS00009367. However, in October 2022, the DRKS changed their policy regarding registrations from outside the EU. As the UK had left the EU, the DRKS deactivated our research registration. The DRKS email confirms it, as follows:
|
12 Oct 2022, 10:57 | |||
|
||||
Dear Dr. Allen,
Your study DRKS00009367 was deactivated. The issue was discussed with the team and will not happen again. Please accept my sincere apologies for the caused inconvenience.
Seeing that we changed our policy regarding Non-EU-Registrations on 1st of October this year, I would kindly ask you to apply registration on any other WHO-primary registry which accepts worldwide registration.
Thank you!
Please let me know in case you have any further questions.
Best regards,
Torben Stodtmeister
Head of DRKS
———————————————————————-
Should you need any further information, please do not hesitate to contact Dr Simon Allen by sending an email to: info@finetreatment.com